Abstract
Introduction:
Success of umbilical cord blood transplantation (UCBT) is limited by an increased risk of graft rejection, mixed chimerism, infection, and delayed immune reconstitution. Additionally, strategies such as donor lymphocyte infusion (DLI) to improve declining donor chimerism or augment immune reconstitution are extremely limited in UCBT. We hypothesized that removing and storing a portion of the thawed cord blood graft for future use as a cord DLI (cDLI) could be used to improve mixed chimerism or augment immunity without adverse impact on engraftment or graft versus host disease (GVHD).
Methods:
Patients with inborn errors of metabolism, immunity, or hematopoiesis underwent a single unit UCBT following a reduced-intensity conditioning regimen of alemtuzumab, hydroxyurea, fludarabine, melphalan, and thiotepa (ClinicalTrials.gov: NCT00744692) with a thaw and dilute (no wash) methodology. Up to 5% of the cord blood graft was removed from the thawed graft and re-cryopreserved for potential cDLI. The cDLI was infused at the discretion of the treating physician in discussion with the study PI (P.S.) in cases of mixed or declining donor chimerism, viral infection, or delayed immune reconstitution.
Results:
A total of 33 patients had cells cryopreserved prior to UCBT for potential cDLI. The characteristics of these patients are shown in Table 1. A total of 18 patients received cDLI at a median of 65 days (range 14-124) post-UCBT. The median cell dose of the cDLI was 8.46e+05 CD3/kg and 1.53e+04 CD34/kg. The indication for cDLI was mixed whole blood or CD3 chimerism (n=9), viral infection (n=9), and delayed immune reconstitution (n=5); 5 patients had more than one indication for cDLI, most commonly infection with delayed immune reconstitution (n=4).
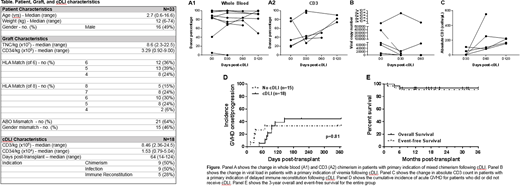
All patients engrafted with neutrophils at a median of 15 days post-transplant (range: 10-31 days); however, one patient experienced secondary graft failure at day 38 post-transplant despite cDLI at day 28 post-transplant. Improvement in chimerism was seen in 5 of 9 patients, viral load and/or clinical symptoms of viral infection in 5 of 9 patients (including gastroenteritis due to rotavirus (n=1) or adenovirus (n=1)), and absolute CD3 count in 5 of 5 patients (Figure).
A total of 15 patients (45%) developed acute grade I-III GVHD at a median of 69 days (range: 13-95 days) post-UCBT. In the group of patients who received cDLI, 8 developed grade I-II skin GVHD at a median of 19 days (range 4-81 days) post-cDLI. In addition, 2 patients with a history of GVHD received subsequent cDLI without GVHD flare post-cDLI and were analyzed for progression post-cDLI. No patients who received cDLI developed grade III or IV acute GVHD or extensive chronic GVHD, while 4 patients who did not receive cDLI developed grade III GVHD affecting skin and/or the gastrointestinal tract. There was no difference in the cumulative incidence of acute GVHD between those who received cDLI and those who did not (44% and 33%, respectively; p=0.81; Figure). The 3-year overall and event-free survival of the entire group was 94% and 91% respectively (Figure), with treatment-related mortality of 6% at 1 year post-transplant.
Conclusions:
The use of cDLI is safe in patients undergoing single-unit UCBT. Despite the removal of a small portion of the cord blood graft for cDLI, all patients engrafted with neutrophils and no increased morbidity was seen. In addition, although the sample size is small, over half of the patients who received cDLI had improvement in the primary indication (chimerism, viral infection, or immune reconstitution) for cDLI, with the most benefit seen in patients receiving cDLI for delayed immune reconstitution, as evidenced by both clinical improvement in infectious symptoms and increases in absolute CD3 count. This suggests that cDLI may be an effective way to address mixed chimerism, infection, and particularly, delayed immune reconstitution and should be explored in a larger clinical trial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal